Activation Energy - Definition, Units, Examples:
Activation energy Definition:
- Activation energy is the energy difference between the ground state conformation and the transition state conformation.
- Activation energy is defined as the minimum amount of extra energy required to initiate the reactions.
What is Activation Energy:
- Every cell in the human body undergoes thousands of chemical reactions every second. All these reactions have their own characteristic activation energy.
- In our body enzymes are responsible for lowering the activation energy.
- They contribute to the easy and fast start of the reaction in our cells.
- Activation energy is the minimum amount of energy required to initiate a chemical reaction.
- The rate of a chemical reaction depends on its activation energy.
- The activation energy can be determined by measuring the rate constant at two temperatures.
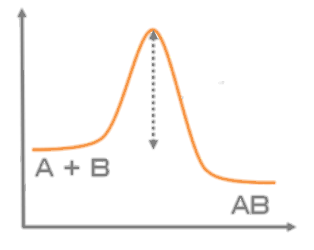
- Let's take the reaction "A + B → AB" as an example.
- In order to make product AB from reactants A and B, it is necessary to pass through a high-energy state called an activated state.
- Activation energy is the energy required to reach that activated state.
What is the SI Unit of Activation Energy?
Activation energy is denoted by Ea. It is usually measured in joules (J) and or kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol).
What would happen if the reactions proceeded without activation energy
Hydrogen and oxygen would turn into water vapor instantly. Metal surfaces would oxidize very quickly, we would instantly digest food, and food would spoil very quickly. Water would react with all gases in the air, nitrogen and oxygen, which are part of the air, would react with each other, turning into toxic and smelly nitric oxide. All molecules would interact instantly. In other words, the perfect order in the ecosystem would be violated.
Frequently Asked Questions on Activation Energy:
1. How do enzymes affect the activation energy of a reaction?
Answer: Like any catalysts, enzymes increase the rate of a chemical reaction by reducing the activation energy (energy barrier) of the reaction. The decrease in the activation energy during enzymatic catalysis is due to an increase in the number of stages of the chemical process.
2. What does the Arrhenius equation show?
Answer: Arrhenius equation is an equation that establishes the relationship between the rate of a chemical reaction and temperature, activation energy, activation entropy and frequency of particle collisions in a system.
3. What is an activated complex?
Answer: A transition state or activated complex is an intermediate state during a chemical reaction in which atoms take on a specific configuration along the reaction coordinate.
4. When is the activation energy zero?
Answer: For recombination reactions of free radicals (including atoms), as well as for a wide class of exothermic ion-molecular reactions , the activation energy is zero or very small compared to typical chemical energies.
5. How to determine the activation energy of a reaction?
Answer: The activation energy E act is determined from the slope of the dependence lg k = f (1/T), obtained by taking the logarithm of the Arrhenius equation (1). To determine the reaction rate constant k, it is necessary to carry out a series of experiments at different temperatures under isothermal conditions with continuous recording of the mass loss.
6. What values can the Activation Energy take?
Answer: In most cases , the activation energy of chemical reactions between neutral molecules ranges from 80 to 240 kJ/mol. The LOWER the activation barrier Ea of any chemical reaction, the FASTER it goes under given conditions, because more molecules A and B are able to overcome the barrier per unit time.