Monomeric Enzymes: Definition, Types, Examples, and Functions:
Hey students! Today we are going to learn about a very special and important area of enzymology – Monomeric Enzymes! As you know, enzymes are biological catalysts, which speed up biological and chemical reactions that take place in living systems. Looking at various enzymes, we see different types of enzymes - monomeric enzymes are the simplest enzymes because they are composed of just one polypeptide chain and can still do catalytic activity. They often follow classical Michaelis-Menten kinetics and have traditionally been used as model enzymes when studying enzymology and biochemistry. Now, let us take the first step to understand what monomeric enzymes are, how they work, and the many examples, applications and relevance of monomeric enzymes in both biology and industry.
Definition of Monomeric Enzymes:
- Enzymes having only one polypeptide chain are called monomeric enzymes.
- Enzymes containing a single polypeptide chain with only one active center are called monomeric enzymes.
What are monomeric Enzymes?
- Enzymes containing a single polypeptide chain with only one active center are called monomeric enzymes.
- They usually contain a single active site in which they may accept substrates and perform catalysis.
- Monomeric enzymes do not require multiple unique subunits for activity like the multimeric enzymes do.
- Examples of monomeric enzymes are trypsin, pepsin, serine, ribonuclease, lysozyme, hexokinase, ribonuclease A, and invertase, etc.
- The relative molecular mass of monomeric enzymes is below 35,000 kDa.
- There are a few monomeric enzymes, and most of them catalyze hydrolysis reactions.
- Monomeric enzymes have primary, secondary, and tertiary structures.
- Monomeric enzymes do not bear a quaternary structure.
- However, some monomeric enzymes are composed of multiple polypeptide chains, such as chymotrypsin, which is composed of three peptide chains, which are connected by disulfide bonds.
- Such monomeric enzymes containing several peptide chains are often generated by the activation and cleavage of a precursor peptide chain.
- Monomeric enzymes are generally easier to study and utilize as model systems for enzymology because their structure is moderate and uncomplicated.
- Because monomeric enzymes cannot be regulated allosterically, they have less regulatory flexibility than multimeric enzymes.
- Monomeric enzymes have medical, biotech, and industrial uses.
- Their main advantages are simplicity of isolation, ease of study, and predictable kinetic behavior.
- Their main disadvantages are limited allosteric regulation and stability.
Examples of Monomeric Enzymes:
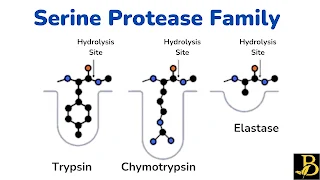
Serine Protease:
- Serine proteases are a family of proteases whose function is to break peptide bonds of proteins.
- In mammals, serine proteases play important roles, especially in digestion, coagulation, and complement systems.
- Its activation is achieved through a set of amino acid residue changes in the active center, one of which must be serine (the origin of its name).
- Three of the enzymes secreted by the pancreas are serine proteases: Chymotrypsin,
- The primary structure (amino acid sequence) and tertiary sequence (spatial arrangement of all atoms) of these three enzymes are similar.
1. Trypsin:
- Trypsin is a proteolytic enzyme that hydrolyzes peptides and proteins, endopeptidase.
- The active site of trypsin consists of serine, arginine, lysine, and a carboxyl group.
- This enzyme is released from the pancreas as a zymogen and converted to the active form in the small intestine.
- Trypsin is synthesized in the pancreas in the form of a proenzyme called trypsinogen and, in this form, as part of pancreatic juice, it enters the duodenum, where an alkaline environment, under the influence of the proteolytic enzyme enterokinase, the hexapeptide is removed from the trypsinogen molecule and a biologically active structure of trypsin is formed.
- After the activation of trypsin by enterokinase, the process of autocatalysis begins and trypsin then acts as an enzyme that activates trypsinogen, chymotrypsinogen, procarboxypeptidase, and other proenzymes of the pancreas.
- Trypsin is active at pH 5.0 to 8.0 with an optimum activity at pH = 7.0.
- Trypsin is an important enzyme for intestinal digestion, which breaks down proteins entering the duodenum of food.
2. Chymotrypsin:
- Chymotrypsin proteolytic enzyme that hydrolyzes the peptides and proteins.
- Chymotrypsin (EC 3.4.21.1) is synthesized in the pancreas in the form of proenzymes called chymotrypsinogen A and chymotrypsinogen B.
- Chymotrypsin A and B have different molecular sizes but similar specificities.
- After synthesis, it enters the duodenum, where, under the influence of trypsin, chymotrypsinogens are converted into α-, β- and π- chymotrypsins.
- Chymotrypsin preferentially cleaves the bonds formed by the COOH groups of amino acids with hydrophobic side chains, especially tyrosine, tryptophan, phenylalanine, and leucine.
- Chymotrypsin is most active at pH 7.5 to 8.2.
- It has a broader specificity of action than trypsin.
3. Elastase:
- Elastase proteolytic enzyme that breaks down peptides and proteins.
- Elastase (EC 3.4.21.36.) catalyzes the hydrolysis of carboxyl-containing polypeptide bonds such as alanine, glycine, isoleucine, leucine, or valine.
- Elastase is also known as pancreatic elastase, elastase 1, pancreatic elastase, or serine elastase.
- It is believed that this enzyme mainly acts on elastin and is so named.
- It is a serine protease secreted by the pancreas in the form of zymogen (elastase) and is involved in protein digestion in the intestine.
- Molecular weight 28,000 daltons.
- Elastase is synthesized in the pancreas in the form of an inactive form - the proenzyme of proelastase, which, as part of the pancreatic juice, enters the duodenum, where, under the influence of trypsin, it turns into elastase.
- Elastase has a broader specificity of action than trypsin.
- Elastase can cleave the protein elastin, which is not hydrolyzed by either trypsin or chymotrypsin.
- Elastase can enter the blood in increased amounts in pancreatitis.
Role of Serine Protease:
- Several activated coagulation factors are serine proteases, including:
- Coagulation factor ten (X), also known as the Stuart-Power factor, is a storage-stable factor involved in the intrinsic and extrinsic coagulation pathways.
- Deficiency can cause systemic coagulation disorders (factor tenth deficiency).
- Coagulation eleventh factor (XI), that is, plasma thrombin precursor: a stabilizing factor acting on the intrinsic coagulation pathway.
- A deficiency of this factor causes a systemic blood-clotting defect, known as hemophilia C or Rosenthal syndrome, similar to classic hemophilia.
- Thrombin comes from prothrombin, the enzyme that converts fibrinogen into fibrin.