Enzymes are biological molecules, that act as catalysts to speed up biochemical reactions in living organisms. They facilitate reactions by lowering the activation energy required for the reaction to occur.
What are Enzymes
- Enzymes are the biocatalysts that enhance the rate of reaction by decreasing the activation energy.
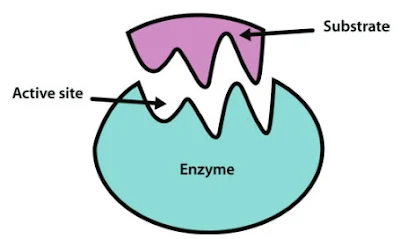
- The active site is a specific region on the enzyme's surface where the substrate (the molecule the enzyme acts upon) binds.
- It has a complementary shape to the substrate and contains amino acid residues that catalyze the chemical reaction.
- Enzymes work by binding to specific substrates at their active sites, forming enzyme-substrate complexes.
- This binding lowers the activation energy needed for the reaction, allowing it to proceed faster. Enzymes remain unchanged after the reaction and can catalyze multiple reactions.
- Enzyme consist of cofactors and coenzyems.
- Cofactors are non-protein molecules or ions that assist enzymes in catalyzing reactions.
- Coenzymes are organic cofactors that often participate in transferring chemical groups between molecules during enzymatic reactions.
- Enzyme activity is influenced by factors such as temperature and pH.
- Each enzyme has an optimal temperature and pH range at which it functions most effectively.
- Extreme temperatures or pH levels can denature enzymes, disrupting their structure and activity.
Basic Structure of Enzymes by X-ray Crystallography
The structure of an enzyme is critical to its function as a biological catalyst. Enzymes are usually large proteins made up of long chains of amino acids. The specific sequence and arrangement of these amino acids determines the unique structure and properties of each enzyme. The detailed structure and components of the enzyme are as follows.
Image Source: wou.edu
Protein composition:
- Enzymes are primarily composed of proteins, which are polymers of amino acids linked by peptide bonds.
- Amino acids are organic molecules consisting of a central carbon atom (alpha carbon) bonded to a hydrogen atom, an amino group (NH2), a carboxyl group (COOH), and different side chains (R groups) depending on the type. of amino acids.
- The sequence of amino acids in the primary structure of an enzyme is determined by the gene encoding that enzyme.
Level of protein structure:
- Primary structure: The linear sequence of amino acids within the polypeptide chain of an enzyme.
- Secondary structure: Local folding patterns within a polypeptide chain, such as alpha helices and beta sheets, stabilized by hydrogen bonds between amino acid residues.
- Tertiary structure: The overall three-dimensional shape of an enzyme resulting from interactions between distant amino acid residues, such as hydrogen bonds, disulfide bonds, hydrophobic interactions, and ionic interactions.
- Quaternary structure (some enzymes): Some enzymes are composed of multiple protein subunits that come together to form a functional enzyme complex.
Active site:
- The active site of an enzyme is the specific region where the substrate (the molecule that undergoes the enzyme-catalyzed reaction) binds.
- The active site is usually a pocket or cleft on the surface of the enzyme that has a complementary shape to the substrate.
- Amino acid residues within the active site contribute to substrate binding and catalysis.
- The active site may undergo conformational changes (induced fit) upon substrate binding to optimize the enzyme-substrate interaction.
Cofactors and coenzymes:
- Some enzymes require additional non-protein components called cofactors or coenzymes for their catalytic activity.
- Cofactors are metal ions (such as zinc, iron, and magnesium) that participate in enzyme-substrate binding or catalysis.
- Coenzymes are organic molecules (vitamins, NAD+, ATP, etc.) that help enzymes perform certain chemical reactions.
Image Source: schoolworkhelper.net
Frequently Asked Questions on X-ray crystallography:
1. What are the 3 structures of enzymes?
Answer: Enzymes have primary, secondary, and tertiary structures:
Primary Structure: The sequence of amino acids in the enzyme's polypeptide chain.
Secondary Structure: Local folding patterns like alpha helices and beta sheets.
Tertiary Structure: The overall 3D shape of the enzyme resulting from interactions between amino acids.
2. What is enzyme structure and properties?
Answer: Enzymes are proteins with specific three-dimensional shapes (tertiary structure) that include an active site where substrates bind. Their properties include catalyzing specific reactions, being highly efficient, and remaining unchanged after the reaction.
3. What is the basic shape of an enzyme?
Answer: Enzymes can have various shapes, but a common basic shape is globular (ball-like) due to their folded tertiary structure. This shape allows enzymes to be soluble and have a compact structure.
4. What is the structural function of an enzyme?
Answer: The structural function of an enzyme is to provide a specific 3D environment (including the active site) that facilitates the binding of substrates and the catalysis of chemical reactions.
5. What is the structure of an enzyme?
Answer: Enzymes are composed of chains of amino acids folded into specific three-dimensional shapes. This structure includes:
- Primary Structure: Linear sequence of amino acids.
- Secondary Structure: Localized folding patterns like alpha helices and beta sheets.
- Tertiary Structure: Overall 3D shape determined by interactions between amino acids.
- Quaternary Structure, consisting of multiple protein subunits.
6. How does X-ray diffraction analysis work?
Answer: X-ray diffraction analysis is based on the phenomenon of X-ray diffraction on crystals. When rays are scattered by individual atoms or electrons, X-ray waves are added (interference). The addition of two cosine curves is laborious and not particularly visual.
7. Where is X-ray diffraction analysis used?
Answer: Metals, alloys, minerals, inorganic and organic compounds, polymers, amorphous materials, liquids and gases, protein molecules, nucleic acids, etc. are studied by X-ray diffraction analysis. This method is most successfully used to establish the atomic structure of crystalline bodies.
8. Why is X-ray analysis needed?
Answer: Using X-ray phase analysis, it is possible to determine the composition of non-metallic inclusions in metals (oxides, sulfides, nitrides, carbides), and the distribution of alloying elements in multiphase alloys.
9. What is studied using X-ray diffraction?
Answer: The study of the structure of crystalline, as well as polycrystalline substances using the phenomenon of diffraction of electromagnetic waves in the X-ray range is the essence of X-ray diffraction analysis. As is known, the structure of the arrangement of atoms in a crystal can have a rather complex, three-dimensional order, determined by the so-called.