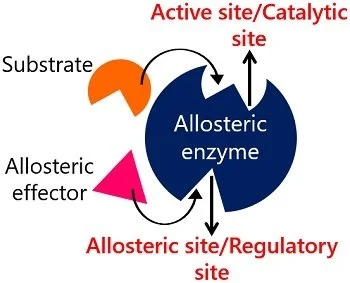
Allosteric enzymes are enzymes that have an additional binding site for effector molecules other than the active site.
Allosteric Enzyme Definition:
- Allosteric enzymes are enzymes that have an additional site, as well as the active site.
- Allosteric enzymes are enzymes that have an additional binding site for effector molecules other than the active site.
What are allosteric enzymes?
Image Source: biologyreader.com
- In addition to containing active centers, allosteric enzymes also have allosteric centers.
- Allosteric center in an enzyme controls the catalytic activity of the active center through the allosteric center.
- Allosteric centers are sites where molecules other than substrates bind, collectively referred to as allosteric effectors.
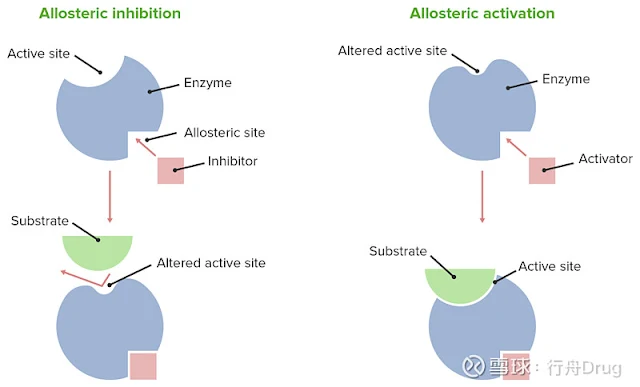
- Specific small molecules can bind to the allosteric center and guide the conformation of the enzyme to change, thereby controlling the activity of the enzyme.
- Various small molecules that bind to the allosteric center are collectively referred to as allosteric effectors or allosteric modulators.
- The substances that activate the enzyme activity are called allosteric activators, and those that inhibit it are called allosteric inhibitors.
- Enzyme activity regulation by allosteric effectors is one of the important means of cellular metabolism regulation.
- The presence of allosteric effectors can alter the kinetic behavior of a typical positive synergistic isomerase toward the substrate.
Read: Functions of Enzymes
Properties of allosteric Enzymes:
- Allosteric enzymes generally contain two or more subunits.
- These enzymes have two sites one is the active site and another is an allosteric site.
- The active center is responsible for substrate binding and catalysis, and the allosteric center is responsible for regulating the enzyme reaction rate.
- The active center and the allosteric center are located on different subunits or on different parts of the same subunit.
- Most allosteric enzymes have more than one active center.
- Some allosteric enzymes have more than one allosteric center and can be regulated by different metabolites.
- Allosteric enzymes do not obey the Michaelis equation and the kinetic curves are not typically hyperbolic.
- The reaction rate of allosteric enzymes is plotted against the substrate concentration, that is, the plot of V against [S] does not obey the Michaelis equation, and the resulting curve is generally not a hyperbolic curve.
- A small change in the substrate concentration, allosteric enzymes can greatly control the reaction rate, which is why allosteric enzymes can sensitively adjust the reaction rate.
- In addition to allosteric inhibitors, allosteric enzymes may also be affected by competitive inhibitors like other non-allosteric enzymes.
- Compared with non-allosteric enzymes, allosteric enzymes are in the minority.
Read: Activation Energy
Features of Allosteric Enzymes:
- Possess allosteric sites, which is the only standard for us to judge whether one enzyme is an allosteric enzyme or not.
- Allosteric effectors bind noncovalently to the enzymes they regulate.
- Allosteric enzymes are generally multisubunit proteins.
- Kinetic curves are generally S-shaped (An allosteric enzyme usually has at least one substrate for which the v0 versus [S] curve is sigmoidal rather than hyperbolic.
- Competitive inhibitors can activate allosteric enzymes at a very low concentration
Read: Enzyme Specificity
Allosteric Enzyme Examples
| Enzyme | Function | Example of Regulation |
|---|---|---|
| Phosphofructokinase | Helps regulate glycolysis | Inhibited by high levels of ATP |
| Allosteric pyruvate kinase | Involved in glycolysis pathway | Activated by fructose-1,6-bisphosphate |
| Allosteric phosphoenolpyruvate carboxylase | Involved in gluconeogenesis | Activated by acetyl-CoA and inhibited by aspartate |
| Allosteric ribonucleotide reductase | Involved in DNA synthesis | Regulated by levels of deoxyribonucleotides |
Allosteric Regulation of Enzymes:
Image Source: xueqiu.com
Frequently Asked Questions on Allosteric Enzymes:
1. What is the allosteric center for?
Answer: The allosteric center (allos - alien) is the center of regulation of enzyme activity, which is spatially separated from the active center and is not present in all enzymes.
2. What is an Enzyme Effector?
Answer: Substances that affect the activity of enzymes are called effectors. These can be inhibitors or activators. Inhibitors are the compounds that inhibit the catalytic process, or activators are the substances that accelerate this process.
3. What are Allosteric Effectors?
Answer: Allosteric effectors are effector molecules that can bind to regulatory proteins involved in RNA transcription in order to change their activity.
4. Why are enzyme inhibitors needed?
Answer: The inhibitor causes conformational changes that prevent the enzyme from converting the substrate into a product, but do not affect the affinity of the enzyme for the substrate.
5. What substances can be enzyme inhibitors?
Answer: Enzyme inhibitors are many drugs of natural or synthetic origin. Metabolites can also be inhibitors of enzymes in regulatory processes.